Backdoor method creates high-entropy material at lower temps
By David Nutt, Cornell Chronicle
Entropy is a hot mess.
Randomness and disorder are not exactly virtues in science. Yet it turns out, a sloppy jumble of differently sized atoms can do a better job stabilizing certain nanocrystals than a tidy arrangement of such elements. These so-called high-entropy materials are now being eagerly studied because they could revolutionize a broad range of applications, from energy storage and conversion to ultra-high temperature thermal insulators and electromagnetic interference shielding.
An interdisciplinary team has developed a backchannel way, using solubility rather than entropy, to overcome thermodynamic constraints and synthesize high-entropy oxide (HEO) nanocrystals at lower temperatures – the same result, but with less mess.
The team’s new paper, “Colloidal Synthesis of Monodisperse High Entropy Spinel Oxide Nanocrystals,” published June 17 in the Journal of the American Chemical Society. The lead author is doctoral student Jonathan Rowell.
The field of high-entropy materials is relatively new, but the interest is so intense, there are practically more review papers on the topic than there are high-entropy materials themselves, according to Richard Robinson, associate professor of materials science and engineering in Cornell Engineering, and the paper’s corresponding author.
“It’s such an exciting domain because high-entropy materials could be better at many applications, like catalysts, than what anybody has ever seen before,” he said. “They have properties beyond what other binary or ternary materials have, because of their ability to mix all of the different wave functions of these many cations and create a lattice that is strained but stable. The problem is that it’s so hard to access uniform-sized nanocrystals of these materials. It’s hard to create them because the standard conditions are just not favorable for controllable growth.”
Until now, high-entropy stabilization in nanocrystals has relied on two actions: adding many elements and elevated temperatures. Crystals are generally composed of atoms that bond together and form a lattice. Adding a few extra atoms of different size and binding strength will cause a well-ordered lattice to fall apart, usually from the strain. But adding five or more different types of atoms – in this case, positively charged ions called cations – along with an increase of temperature in the entropy term creates just the right conditions to stabilize the formation energy.
“It’s counterintuitive to have a material so randomized it is actually stable, which makes it all the more fascinating,” Robinson said. “You always think of building a material with structural and compositional stability, where everything has to slowly come together and be thermodynamically stable. But it turns out, in these high-entropy materials this thermodynamic stability comes from the disorder, which goes against what we’re usually taught. So it’s a delicious idea from a chemistry and material science point of view.”
Rowell discovered that instead of relying on thermodynamics, he could use solubility to create high-entropy particles. Essentially, the researchers precipitated the nanocrystals during colloidal synthesis through an esterification reaction.
“We wanted to take advantage of the low solubility of metal oxide particles – the idea that once an oxide nanocrystal forms, it won’t dissolve back,” Rowell said. “To get there, we needed a reaction that allowed us to control the reaction rate. This led us to this esterification reaction, where the reaction is mostly determined by the acidity of the metal. Since we can look up these acidity values, we can target specific metals, making the synthesis of high-entropy oxides predictable and efficient.”
The most difficult part, however, was the synthesis.
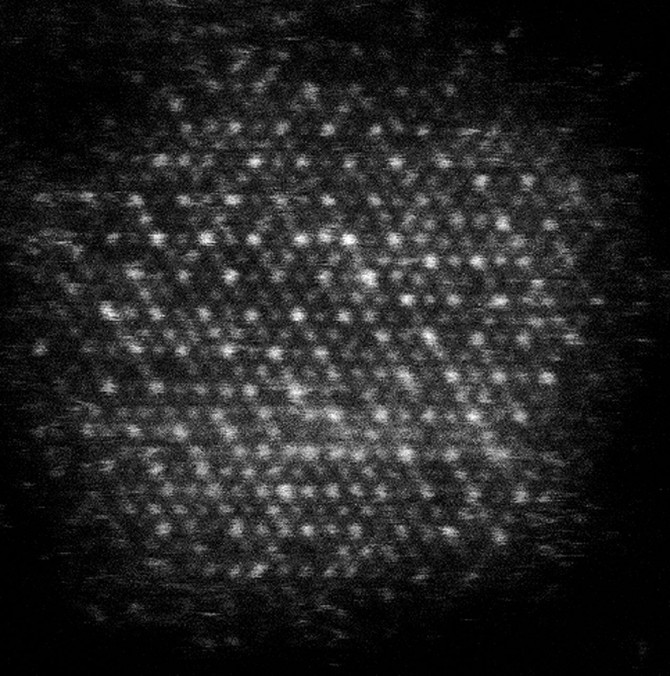
“Usually when you try to make these high-entropy nanocrystals, you grow a lot of particles with many different sizes and nothing is uniform,” Robinson said. “In order to understand the physics and the chemistry and the material science, you really want every single particle to look almost identical. It took Jon a lot of time to refine the reaction to create this monodisperse product.”
The team worked with David Muller, the Samuel B. Eckert Professor of Engineering, who used electron microscopy to confirm the team had created a high-entropy multi-metal with all the cations mixed together in the same lattice, without any elements separating out. Héctor D. Abruña, the Émile M. Chamot Professor of Chemistry in the College of Arts and Sciences, tested the material’s catalytic function, i.e., the ability to accelerate chemical reactions, in alkaline media and found it to be high-performing and stable.
The fact that the nanoparticles contain a variety of cations with different surfaces can potentially make them strong electrocatalysts for fuel cells and batteries, according to Robinson.
“The icing on the cake is the fact that our method is using this backdoor route, so we are not limited by the high-entropy stabilization effect. We can make multi-metal oxides with any number of cations,” he said. “This synthesis opens up the door for creating many versions of multi-metal materials.”
Co-authors include Muller and Abruña; doctoral students Minsoo Kang and Dasol Yoon; and Kevin Jiang, M.S. ’23 and Yafu Jia , M.S. ’23.
The research was supported as part of the Center for Alkaline Based Energy Solutions, an Energy Frontier Research Center funded by the U.S. Department of Energy’s Office of Science, Basic Energy Sciences; and the National Science Foundation (NSF).
The researchers made use of the Cornell Center for Materials Research.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe